For professionals navigating the highly regulated medical device industry, demonstrating proven expertise in quality auditing is the cornerstone of a successful career. The ASQ Certified Medical Device Auditor (CMDA) certification is the definitive credential that validates your skills, enhances your professional credibility, and positions you as a leader in ensuring safety and compliance. This guide provides a comprehensive, step-by-step blueprint to navigate your journey from confirming eligibility to confidently passing the exam.
Achieving this certification signals a deep commitment to quality standards and a mastery of the complex auditing processes unique to medical devices. It is a mark of professional pride and a direct investment in your career trajectory within a globally critical sector.
Why Do Professionals Choose the CMDA ASQ Certification?
Pursuing the CMDA certification is a strategic career move. It goes beyond a line on a resume; it's a testament to your ability to uphold the most stringent quality and safety protocols. Professionals who hold this credential unlock significant advantages:
-
Enhanced Career Opportunities: Certified auditors are highly sought after for roles in quality assurance, regulatory affairs, compliance, and senior management within medical device manufacturing and consulting firms.
-
Increased Earning Potential: The specialized knowledge validated by the CMDA often translates to higher salary benchmarks and more lucrative contract opportunities.
-
Global Recognition: ASQ (American Society for Quality) certifications are respected worldwide, providing you with a portable and internationally recognized credential.
-
Demonstrated Compliance Mastery: You will possess a verifiable understanding of key regulations and standards, such as FDA 21 CFR 820, ISO 13485, and the Medical Device Single Audit Program (MDSAP).
-
Improved Auditing Performance: The preparation process itself deepens your knowledge, making you a more effective, efficient, and insightful auditor capable of driving real improvements within an organization.
Are You Eligible? Understanding the CMDA Requirements
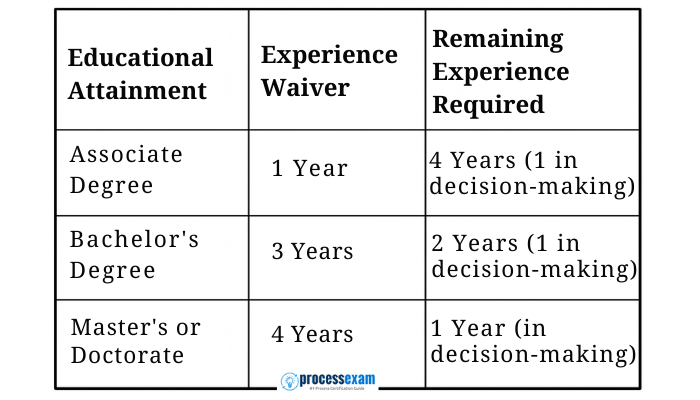
Before embarking on your preparation, the first step is to confirm your eligibility. ASQ has established clear criteria based on professional experience, with waivers available for formal education.
You must have five years of on-the-job experience in one or more areas of the Certified Medical Device Auditor Body of Knowledge. A critical component of this is that at least one year of that experience must have been in a "decision-making" role. This means you were responsible for managing, directing, or making critical judgments within a quality or auditing framework.
ASQ offers waivers that can reduce the five-year experience requirement:
-
Key Takeaway: The "decision-making" year is non-negotiable. Ensure your work history clearly reflects this responsibility before applying for the CMDA certification exam.
A Closer Look at the CMDA Exam Structure
Knowing the exam format is essential for building an effective study plan. The CMDA exam is designed to test both your theoretical knowledge and your practical application of auditing principles in the medical device context.
-
Name: ASQ Certified Medical Device Auditor
-
Exam Code: CMDA
-
Exam Format: 145 Multiple-Choice Questions
-
Duration: 4 hours and 18 minutes (Total appointment time is 4.5 hours)
-
Passing Score: 550 out of a possible 750
-
Exam Policy: Open Book.
-
CMDA ASQ Certification Cost: Member: $433 USD | Non-member: $533 USD | Retakes: $333 USD
Step 1: Build a Preparation Plan Based on the Exam Syllabus
A structured preparation plan is your roadmap to success. The ASQ CMDA Body of Knowledge (BoK) is divided into five key domains, each with a specific number of questions on the exam. Your study time should be allocated proportionally.
-
Auditing Fundamentals (12 Questions): Covers audit types (product, process, system), terminology, auditor ethics, and roles/responsibilities.
-
Auditing and Inspection Processes (28 Questions): Focuses on the entire audit lifecycle: planning, execution, reporting, follow-up, and closure.
-
Medical Device Quality Management System Requirements (38 Questions): This major section tests your knowledge of standards and regulations like ISO 13485 and FDA QSR (21 CFR 820).
-
Technical Medical Device Knowledge (42 Questions): The largest section. It includes sterilization, risk management (ISO 14971), biocompatibility, clinical data, and device-specific processes.
-
Quality Tools and Techniques (15 Questions): Involves statistical analysis, sampling plans, root cause analysis, and other problem-solving methodologies.
Actionable Tip: Create a study calendar that allocates more time to the higher-weight sections. A balanced approach that covers all five domains is crucial for achieving the 550-point passing score.
Step 2: Focus on High-Weight Sections (Technical Knowledge & QMS)
With 80 out of 145 questions coming from just two sections - Technical Medical Device Knowledge (42) and QMS Requirements (38) - it is clear where your primary focus should be. Mastering these domains is essential for passing the CMDA exam.
-
For QMS Requirements: Don't just memorize clauses from ISO 13485 or FDA 21 CFR 820. Understand their intent. How does a CAPA system function in practice? What are the key elements of design controls? Focus on the "why" behind the regulations.
-
For Technical Knowledge: This area is broad. Identify your weaker points. If you have less experience with sterilization validation, dedicate extra time to it. Become intimately familiar with the principles of risk management as defined in ISO 14971, as it is a central theme in modern medical device auditing.
Step 3: Leverage ASQ CMDA Practice Tests and Question Banks
Reading reference materials is only half the battle. To succeed, you must simulate the exam environment. This is where practice exams become your most valuable tool.
Engaging with an ASQ CMDA question bank helps you:
-
Identify Knowledge Gaps: You will quickly discover which syllabus areas require more study.
-
Improve Time Management: With 145 questions in 258 minutes, you have approximately 1.78 minutes per question. Practice tests train you to work efficiently and confidently under pressure.
-
Understand Question Formatting: ASQ questions are designed to test application, not just recall. Practice helps you decipher what is truly being asked.
Investing in a high-quality ASQ CMDA practice test is one of the most effective ways to build confidence and validate your readiness. Look for resources that provide detailed explanations for each answer, helping you learn from your mistakes.
Step 4: Deepen Your Understanding of Compliance and Auditing Fundamentals
While the technical sections are heavily weighted, a weak foundation in auditing fundamentals can undermine your performance. This domain (Auditing and Inspection Processes) contains 28 questions and is critical for success.
Focus on the practicalities of the audit process:
-
Audit Planning: How do you develop an effective audit plan and checklist?
-
Evidence Collection: What constitutes objective evidence? How do you conduct effective interviews and document observations?
-
Non-conformity Reporting: How do you write clear, concise, and evidence-based non-conformance reports?
-
Follow-up and Closure: How do you verify the effectiveness of corrective actions?
The best reference for this is The ASQ Certified Medical Device Auditor Handbook, Fourth Edition. While searching for a free PDF might be tempting, investing in the official handbook from a legitimate source like the ASQ website ensures you have the most accurate and up-to-date information.
Step 5: Your Final-Week Preparation Checklist
The week before the CMDA exam should be about consolidation, review, and mental preparation, not cramming new material.
-
[ ] Review Your Notes: Focus on your summary sheets, particularly for the high-weight sections.
-
[ ] Take One Final Timed Practice Exam: Use this to fine-tune your pacing and identify any lingering weak spots. Analyze the results thoroughly.
-
[ ] Organize Your Open-Book Materials: Tab your handbooks (like ISO 13485 and the CMDA Handbook) for quick reference. Do not plan to look up every answer; your books are for confirming specific details, not for learning concepts during the exam.
-
[ ] Confirm Exam Logistics: Double-check the time, date, and location of your testing center. Plan your route and know the facility's rules.
-
[ ] Rest and Relax: Get adequate sleep in the nights leading up to the exam. A well-rested mind performs significantly better.
-
[ ] Prepare Your Exam Day Kit: Pack your ID, exam confirmation, approved reference materials, and any other permitted items the night before.
ASQ Medical Device Auditor Exam Day Tips for Confidence and Compliance Credibility
Your preparation has led to this day. Walk in with the confidence that you are ready to demonstrate your expertise.
-
Manage the Clock: Keep an eye on the time. If a question is too difficult, flag it and return to it later. It is better to answer all the questions you know first.
-
Use Your References Strategically: The open-book policy is a safety net, not a crutch. Use it to verify formulas, specific clause numbers, or definitions you are unsure of. Wasting time looking up simple answers will hurt your pace.
-
Read Every Question Carefully: Pay close attention to keywords like "NOT," "ALWAYS," or "BEST." Misreading a question is a common and avoidable mistake.
-
Trust Your Preparation: You have put in the work. Trust your knowledge and instincts. Your first answer is often the correct one, so avoid second-guessing yourself unless you have a clear reason to change it.
Start Your CMDA Certification Journey Today
Earning your CMDA certification is a powerful statement about your dedication to excellence in the medical device industry. It is a rigorous but achievable goal that opens doors to new opportunities and cements your status as a trusted expert. By following this structured blueprint, you can approach the exam with a clear plan, a deep understanding of the material, and the confidence to succeed.
Ready to validate your skills and take the next step in your career? Begin your final preparation with comprehensive tools designed for success. Explore the ProcessExam ASQ CMDA certification exam sample questions to get a feel for the exam format and difficulty.